Reports
SliceVault provides a suite of downloadable reports that allow study staff to track progress, verify compliance, and export structured data. Reports are available in Excel (.xlsx) and CSV (.csv) formats, and access depends on the user role and project configuration.
- Click on the menu and choose Reports.
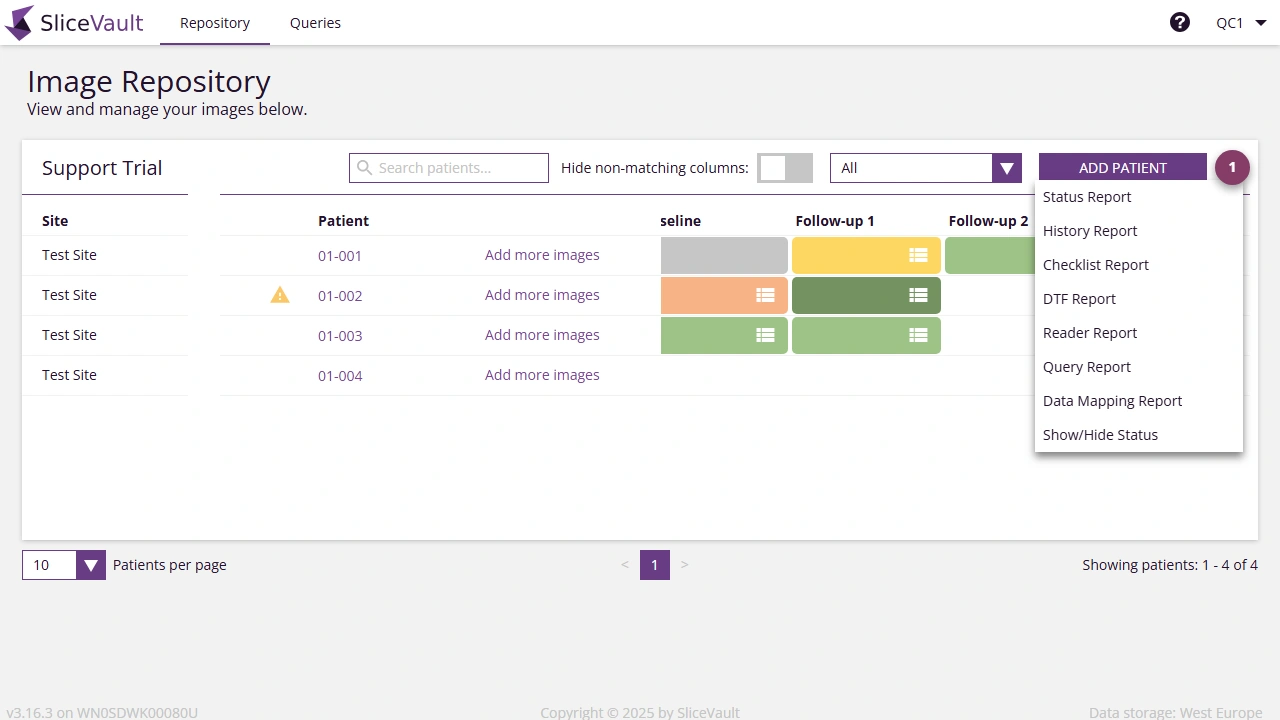
Available Reports
The following reports are avilable for download.
| Report Type | Description | Typical Users |
|---|---|---|
| Audit Log Report | Captures a full audit trail of all actions, including audit ID, timestamp, site, user, user type, patient ID, target, action, and change details. | Trial Administrators |
| User Report | Lists all users in the project, with email, name, roles, site, registration date, last login, and access revoked date. Useful for monitoring access and auditing permissions. | Trial Administrators |
| Checklist Report | Summarizes data from QC or Reader checklists. Includes patient ID, visit, and study-specific fields. | QC Managers, Readers |
| Image Status Report | Provides an overview of upload and review status for each patient and visit, including QC/reader status. | QC Managers, Monitors, Project Managers |
| History Report | Tracks status changes over time. Includes patient ID, visit, category, timestamp, user, and action. | QC Managers, Monitors, Project Managers |
| Query Report | Compiles all queries, with query ID, patient ID, visit, author, replies, verification status, and timestamps. | QC Managers, Monitors, Project Managers |
| DTF Report | Extracts completed Data Transfer Forms. Includes patient ID, visit, submitter, and study-specific DTF fields. | QC Managers, Monitors, Project Managers |
| Reader Report | Summarizes reader assessments, including patient ID, visit, submitter, and study-specific scoring fields. | QC Managers, Project Managers |
| Data Mapping Report | Provides mappings between SliceVault keys and study identifiers. Includes trial key, site key, patient key, visit key, folder key, UIDs, series descriptions, and modality. | QC Managers, Project Managers |
| External Tracking Report | Captures submissions and configurable study fields for external stakeholders. Includes site, patient ID, visit, submission date, and form data. | QC Managers, Project Managers |
| Internal Tracking Report | A comprehensive operational report with patient/visit data, DTF fields, queries, QC statuses, reader results, and adjudication details. | QC Managers, Project Managers |
Each report type serves a different purpose, from compliance (Audit Log, User Report) to operational oversight (Image Status, History, Internal Tracking) and study-specific documentation (DTF, Reader, Checklist).
Note:
- Not all reports are available in every study. The set of enabled reports depends on study configuration and user role permissions.
- Reports are generated twice per day. To ensure you are working with the most recent data, users are recommended to update the report before export.